KamTek is your trusted partner for the complete biospecimen lifecycle from sample receipt to Shipment or Safe disposal. From the moment a sample is received to secure storage, processing, distribution, and digital tracking—we handle it all.
Our integrated services help research institutions, biopharma, clinical trial sponsors, and government organizations simplify operations and protect sample integrity at every stage.

At KamTek, the lifecycle of every biospecimen begins with a secure and documented receipt process. Whether a sample is arriving for long-term Bio storage, laboratory processing, or distribution to clinical trial sites, our sample intake procedures serve as both a checkpoint and a compass—carefully inspecting, verifying, and digitally logging each item.


At KamTek, our biorepository services deliver the infrastructure, expertise, and compliance needed to securely store and manage biological materials and drug products. From large-scale sample storage to ICH stability studies, we provide fully monitored and cGMP-compliant environments tailored to your needs.

Secure storage at RT, 4°C, -20°C, -80°C, and LN2 (-170°C and -196°C) and 24/7 monitoring, redundant backups and automated alerts.
.svg)
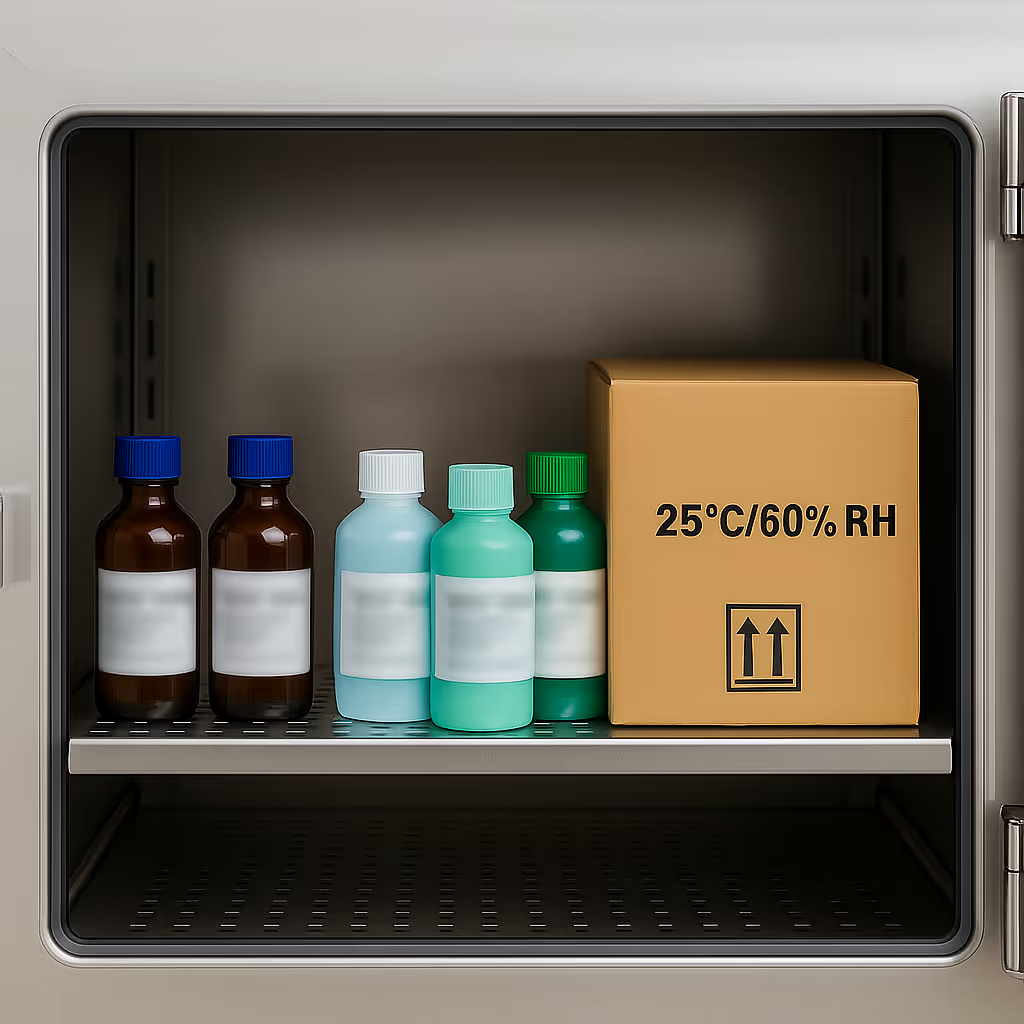
ICH Q1A (R2) guided Stability storage at 25°C/60% RH, 30°C/65% RH, 40°C/75% RH, and more. Backup stability chambers and customizable testing protocols.
.svg)
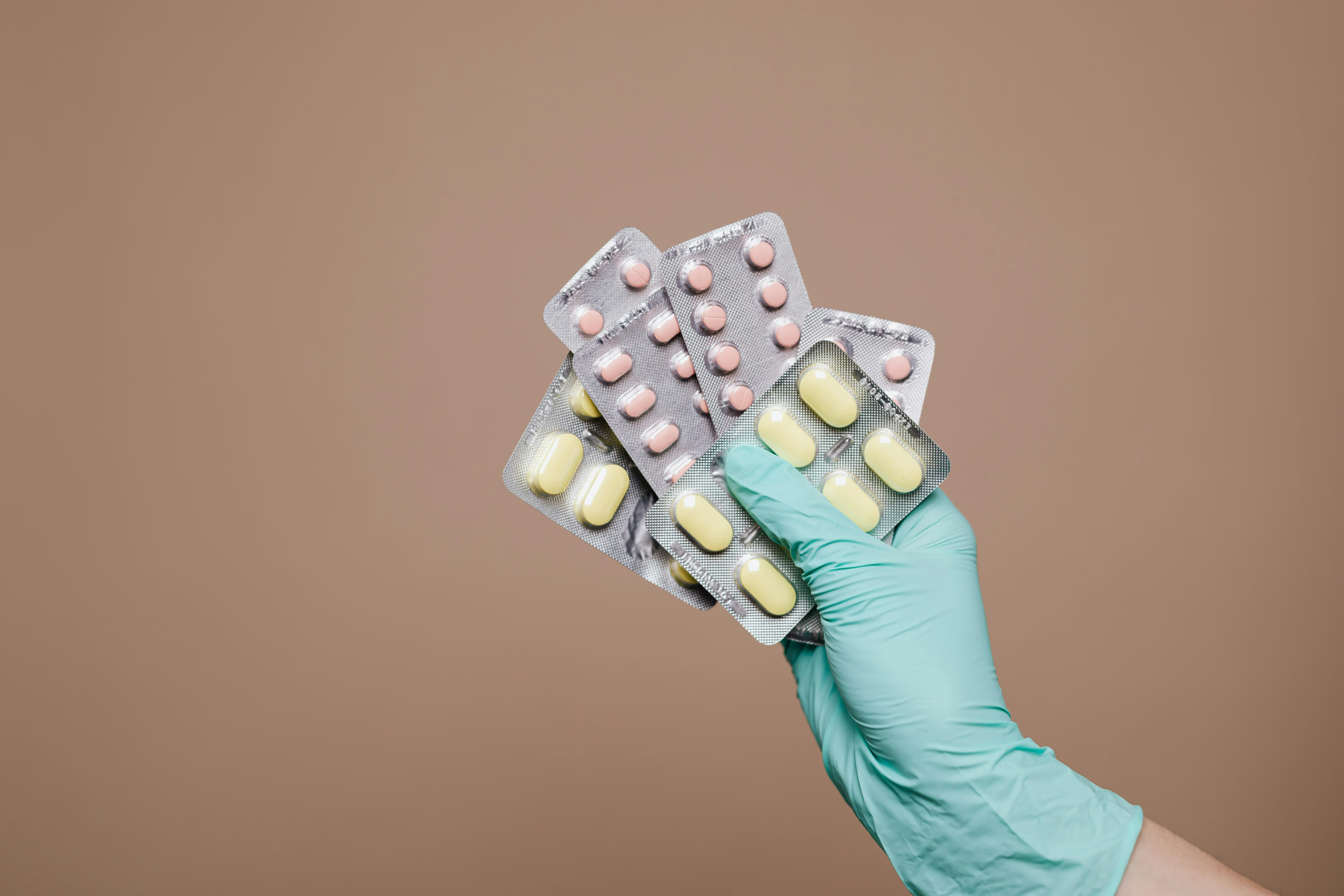
ICH Q1B guided Photo Stability storage with day light exposure of ≥1.2 million lux-hours and near UV light of ≥200Wh/m² at controlled temperature and humidity conditions.
.svg)

KamTek provides precise, compliant sample processing at in house advanced laboratories for research and clinical applications. From cells and nucleic acids to microbes and biospecimens, our end-to-end services ensure your materials are accurately handled, processed, documented, and ready for next steps.
Handling, expanding, processing, and storing live cell materials for research and clinical applications.
Nucleic acid extraction, DNA/RNA stabilization to assays and analysis
Microbiological sample handling and preparation under biosafety-compliant conditions.
High-throughput and custom biospecimen processing with documented traceability and precision.

From inventory to international shipping, KamTek ensures your materials are secure, traceable, and compliant.
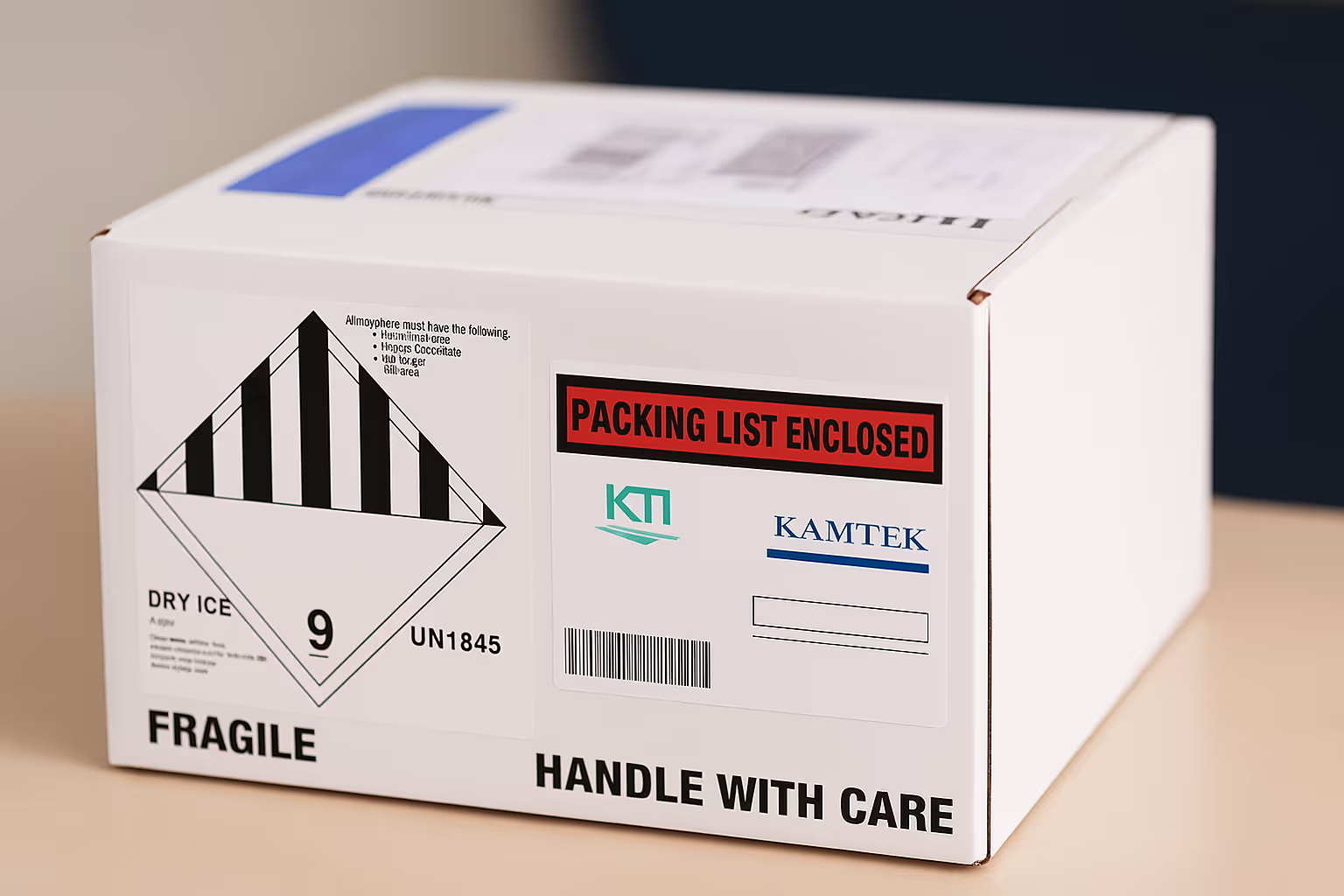
Secure, DOT, IATA compliant shipping of biospecimens and clinical materials across the globe, including frozen, ambient, and cryogenic shipments.
.svg)
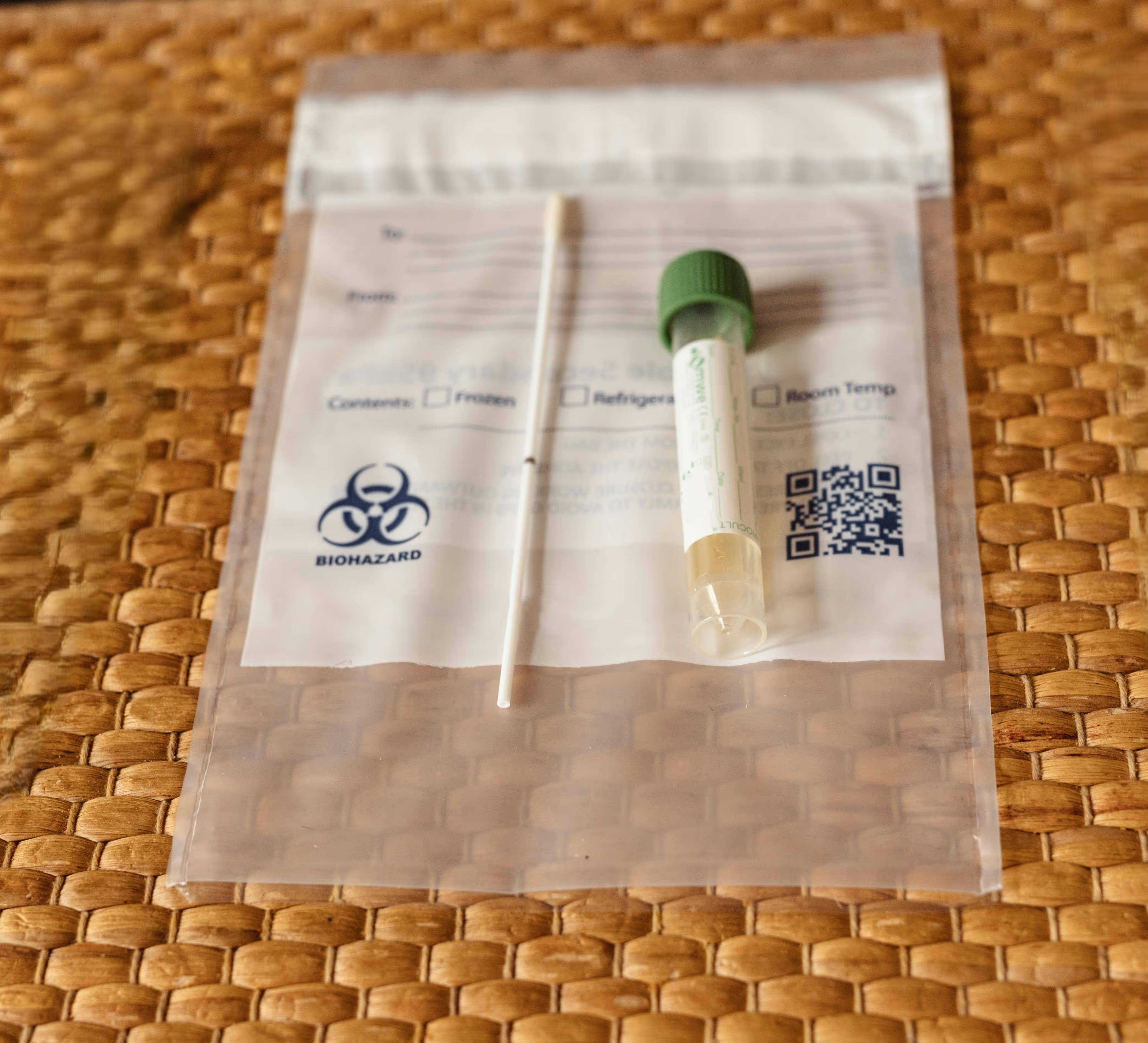
End-to-end custom kitting for clinical trials, research, and diagnostic applications—from sourcing and assembly to labeling, QC, and global distribution.
.svg)

Accurate organized inventory from vials and slides to entire freezers, we catalog, verify, and digitize your materials using secure systems—delivering clear, audit-ready data every time.
.svg)

Secure, compliant disposal of biological samples, freezers, documents, and hazardous materials. From validated autoclaving of biospecimens to document shredding and freezer decontamination, we handle the entire process—including regulatory consultation and certified destruction reports—so you don’t have to.

Biological samples, confidential documents, formalin waste, surplus lab equipment, and mixed waste.
.svg)
At KamTek, we’re more than a storage provider — we’re your end-to-end biospecimen partner.
Our services are designed to simplify your operations, reduce vendor fragmentation, and ensure complete visibility and control across the entire lifecycle of your samples.
From sample receipt to secure disposal, everything happens under one roof — reducing handoffs, delays, and risks.
We tailor processes and protocols to your exact project, sample type, or regulatory requirement.
Biotech companies, clinical trial sponsors, academic labs, and government agencies like FDA, NIH, JHU, ATTC rely on KamTek’s precision and reliability.
FDA-registered, cGMP-compliant, ISO 9001:2015 certified, and aligned with ICH, DOT, IATA, and EPA standards — so you're always inspection-ready.
Secure digital tracking, custom inventory and quality system databases, and integrated reporting give you full control and real-time access.
From on boarding to daily logistics, you’ll have direct access to our experienced team—no call centers, just partners.
From storage and sample processing to global shipping and secure disposal — KamTek handles it all. We are built to scale and respond.
Let us streamline your operations, contact us today.