Bio storage at KamTek involves meticulous receipt, verification, inventory, storage and retrieval of bio samples.
We meet your storage goals for:
Each sample has unique storage requirements to maintain its integrity, and KamTek specializes in providing tailored solutions.
By optimizing the way samples are handled, we minimize their exposure to sub optimal temperatures, ensuring that their characteristics are preserved.
Additionally, KamTek maintains a strict chain of custody for every sample, safeguarding not only the sample itself but also its associated metadata.
At KamTek, we specialize in the storage of biological products at a diverse range of temperatures to meet your specific needs.
Our state-of-the-art facilities are designed to ensure the safety, integrity, and accessibility of your valuable materials.

Our air-conditioned spaces are strictly controlled for temperature and humidity, ensuring the safe storage of a variety of samples such as tissue blocks, glass slides, documents, and specimens in formaldehyde.

We offer walk-in cold rooms and refrigerated units that are temperature-controlled and monitored to store samples at 4°C and -20°C.

Our facility includes both dedicated and shared ultra-low freezers for client materials. We also provide maintenance services for client-owned ultra-low freezers.

State-of-the-art liquid nitrogen tanks are available for vapor-phase or liquid-phase storage with backup bulk supply. Comprehensive maintenance support is also available for client-owned liquid nitrogen storage systems.

When a client notifies KamTek about incoming samples, a sample receipt workflow is initiated.
Upon arrival, packages are brought to the sample receipt station and a visual check is performed to identify any damage, leakage, or discrepancies between the sample and the electronic manifest.
If any issues are identified, the client is contacted immediately to resolve them. Temperature data is retrieved from data loggers
A sample receipt record is then sent to the client, confirming all shipment details.

Products are temporarily stored in the client’s freezer if a location is pre-determined.
If not, the products are placed in a quarantine freezer based on their temperature requirements until the inventory process is completed or final location is assigned.


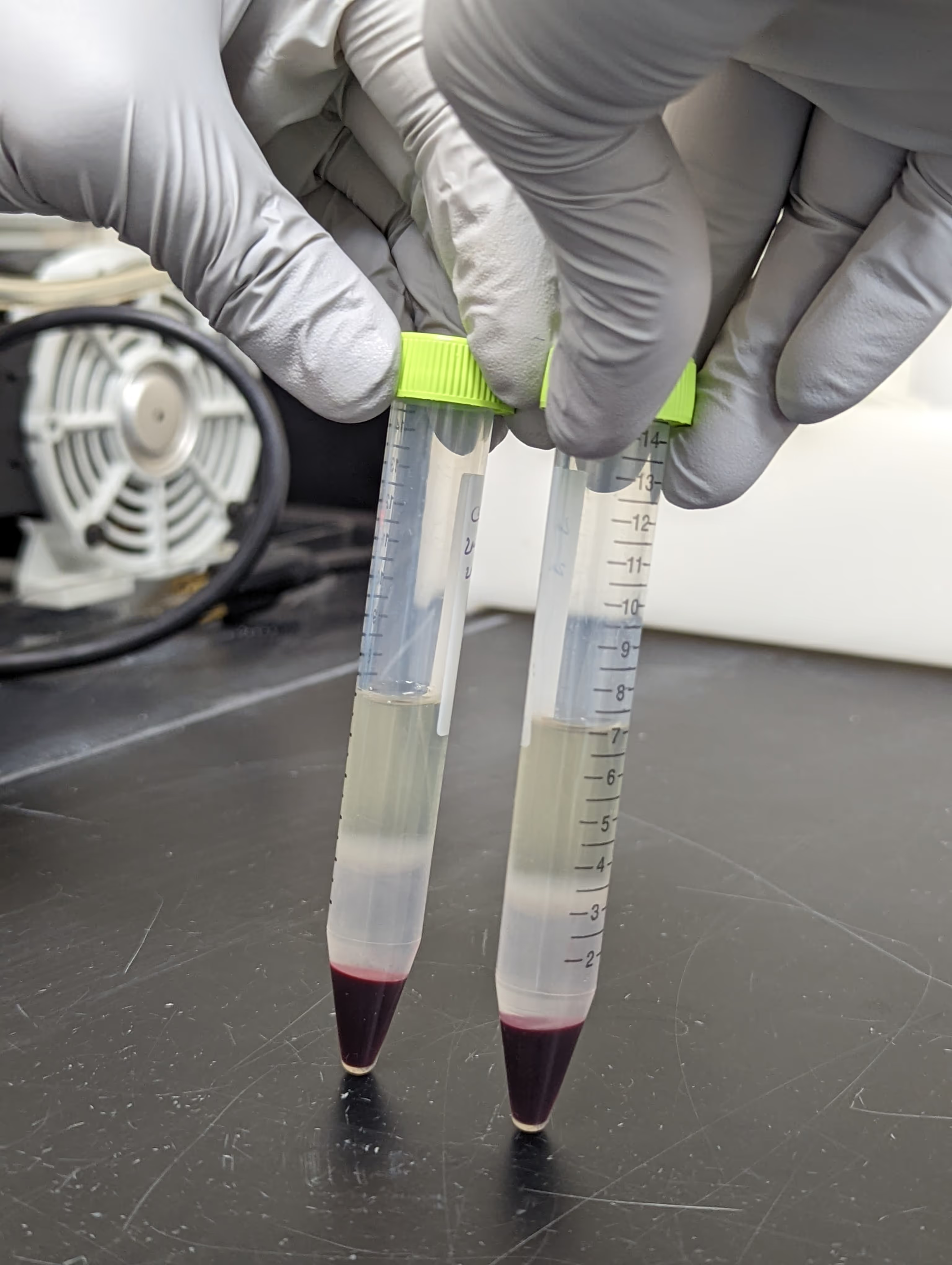
During the inventory process, samples are retrieved carefully using specimen withdrawal containers held and monitored at required temperatures.
Each sample or vial is carefully examined, and the data is entered into a spreadsheet or Inventory database system.
Any discrepancies are reported to the client immediately.
Once the inventory process is complete, the products are safely transferred to their pre-determined storage locations.


After the inventory, the samples are stored in the respective client freezer or KamTek freezer for long term storage, further processing or shipments.
Client will stay in loop during the entire process.


With over 30 years of experience, KamTek is a trusted partner for institutions like the FDA, NIH, and leading research organizations. To date, KamTek has successfully handled over 20 million samples without compromising quality or security.

Our facility features:
- 24/7 monitoring and surveillance.
- Climate-controlled environments.
- Robust backup systems, including LN2 bulk tanks and generators.
- Regulated data access and backups for continuation

KamTek is strategically located within one hour driving distance from NIH, FDA, Johns Hopkins, University of Maryland, NIST, Walter Reed, NCI and many more private companies.

KamTek’s disaster recovery plan ensures your samples are protected even in critical situations. Simulated disaster tests are conducted monthly, guaranteeing readiness at all times.

- Rees monitoring systems track freezer temperatures round the clock, with automated alerts for emergency action.
- Strict visitor and employee access policies to ensure secure handling and storage.
- Dedicated staff available 24/7 to address any issues immediately.
-Advanced sensors (motion, glass-break, fire, flood, burglary etc.) and AI cameras safeguarding the facility 24/7
-Uninterrupted communication system
.svg)
KamTek offers secure storage across multiple temperature ranges: room temperature (RT), 4 °C (refrigerated), –20 °C, –80 °C (ultra-low), and liquid nitrogen vapor phase (–170 °C to –196 °C).
.svg)
We operate a fully regulated, audit-ready environment:
1. FDA 21 CFR Part 211 / cGMP-compliant facility.
2. ISO 9001:2015 certified quality system.
3. Electronic records & signatures (21 CFR Part 11) for tracking sample custody.
4. Continuous monitoring of storage environments with redundant alerts and backup power.
.svg)
Upon receipt:
1. Samples go through a receipt workflow and are accessioned, logged into our secure inventory system.
2. Storage location is defined (based on chosen temperature tier) and chain-of-custody initiated.
3. Environmental conditions are monitored continuously; any excursions trigger automated alerts and client notifications.
.svg)
Yes. Clients retain full ownership and access to their stored materials. We sign a mutual NDA protecting your IP. You can schedule retrievals or shipments as needed. All withdrawals are documented through our chain-of-custody system and we coordinate logistics (on-site pickup or shipment) as requested.
.svg)
We provide:
1. Inventory lists with sample identifiers, storage status, and location mapping.
2. Environmental logs and audit reports upon request.
3. Optional certificate of storage verifying conditions, start date, and sample status for your regulatory records.
.svg)
Our facility is designed for resilience:
1. Backup power (generator/UPS) ensures continuous operation of freezers and LN₂ systems.
2. Disaster recovery protocols are tested and practiced regularly.
3. 24/7 facility monitoring, intrusion alarms, and environmental sensors alert staff immediately of any deviation.
.svg)
We support a wide range of clients—pharmaceutical companies, biotech firms, research institutions, government agencies—and handle materials including: cells, tissues, microbiological samples, diagnostics materials, frozen biobanks, reagents, and more.
.svg)
Pricing depends on multiple factors: sample volume/quantity, desired storage temperature tier, retrieval frequency, special handling requirements (biosafety level, containment), and any value-added services (aliquoting, barcoding, shipment logistics). We provide a formal quote after scope definition.
KamTek’s validated biorepository solutions are trusted by leading research institutions, government agencies, and biopharma partners. Whether you need ambient or cryogenic storage, our team ensures your samples are protected, monitored, and fully compliant—24/7.