With decades of logistics expertise, KamTek supports both domestic and international shipments of critical research and development materials with a short lead time and consistent reliability.
KamTek is fully equipped to ship:

Compliance with DOT, IATA/ICAO, and 49 CFR shipping standards
Shipments at RT, 4°C, -20°C, -80°C, and liquid nitrogen vapor phase in respective approved shipping containers.
Ample dry ice, temperature/humidity data loggers, and real-time monitoring to ensure compliance and sample stability.
Coordinated shipments to and from global trial and research sites.
2-day lead time between request and shipment; emergency and after-hours support available.
Every shipment made following strict SOPs, tracked, recorded, and verified via eQMS software.

.svg)
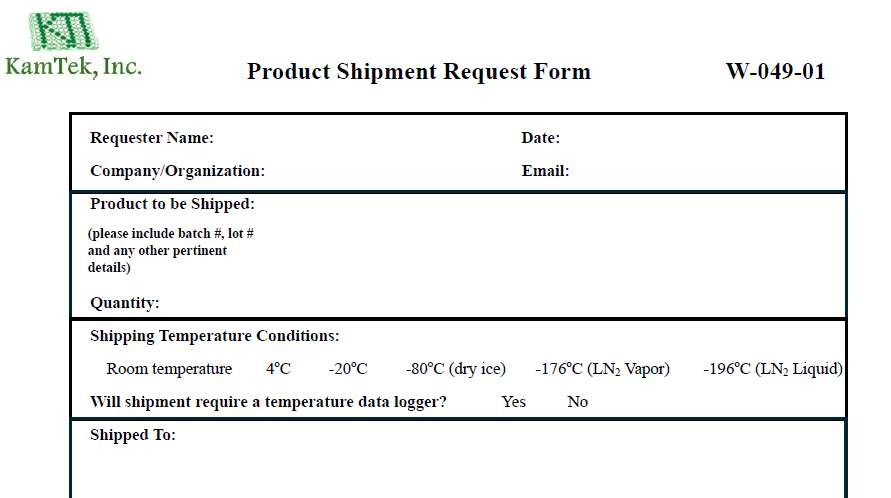
Clients initiate the process by submitting a product shipment request form.
Only 2 day notice is required for most shipments.
.svg)
A digital workflow is created in KamTek’s eQMS software for this request, timestamping every step and ensuring full accountability.

.svg)
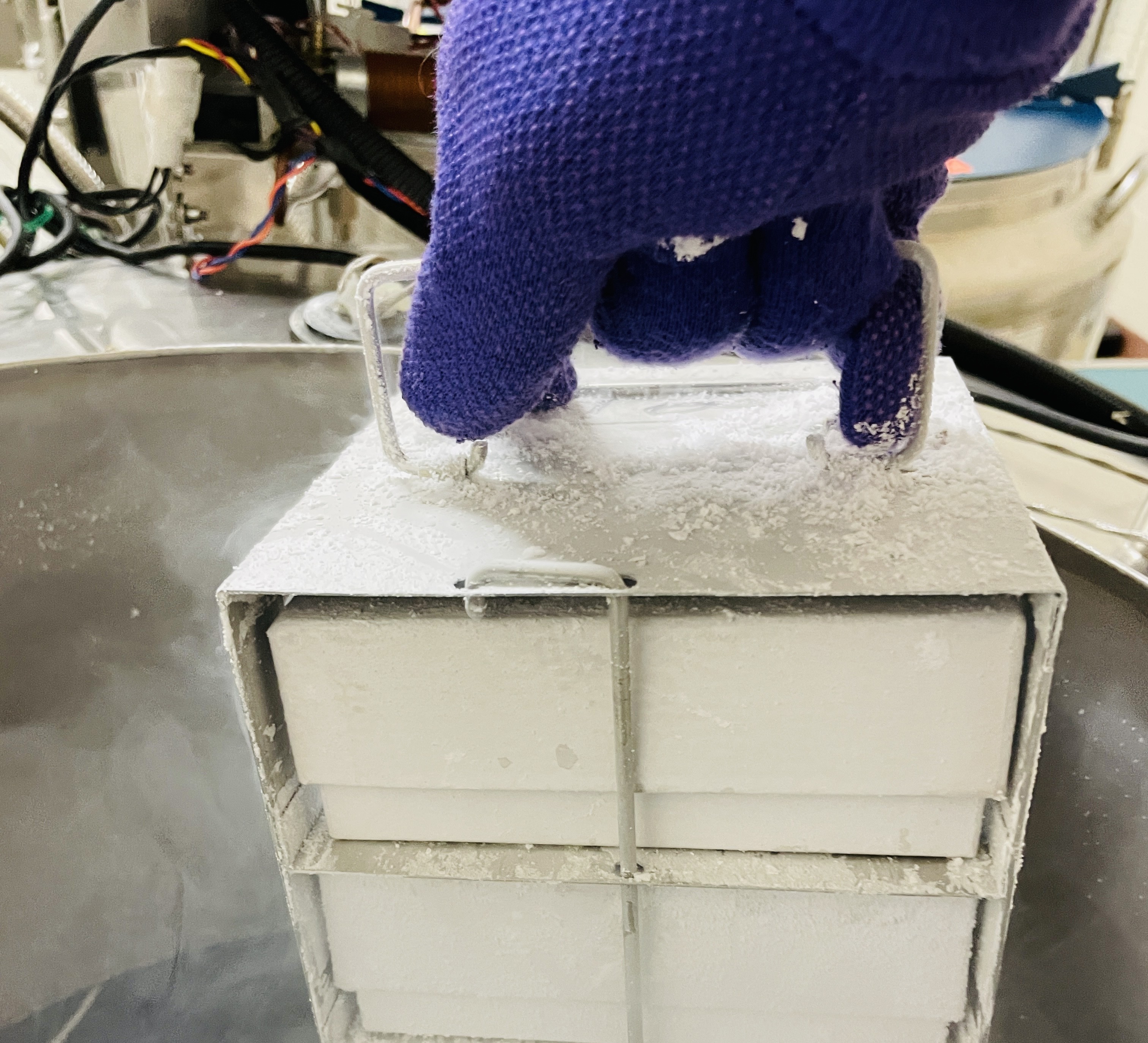
Samples are carefully withdrawn from their storage locations using validated 'specimen withdrawal containers' maintained at the optimal temperature required for the samples (RT/4oC/-20oC/-80oC/LN2).
.svg)
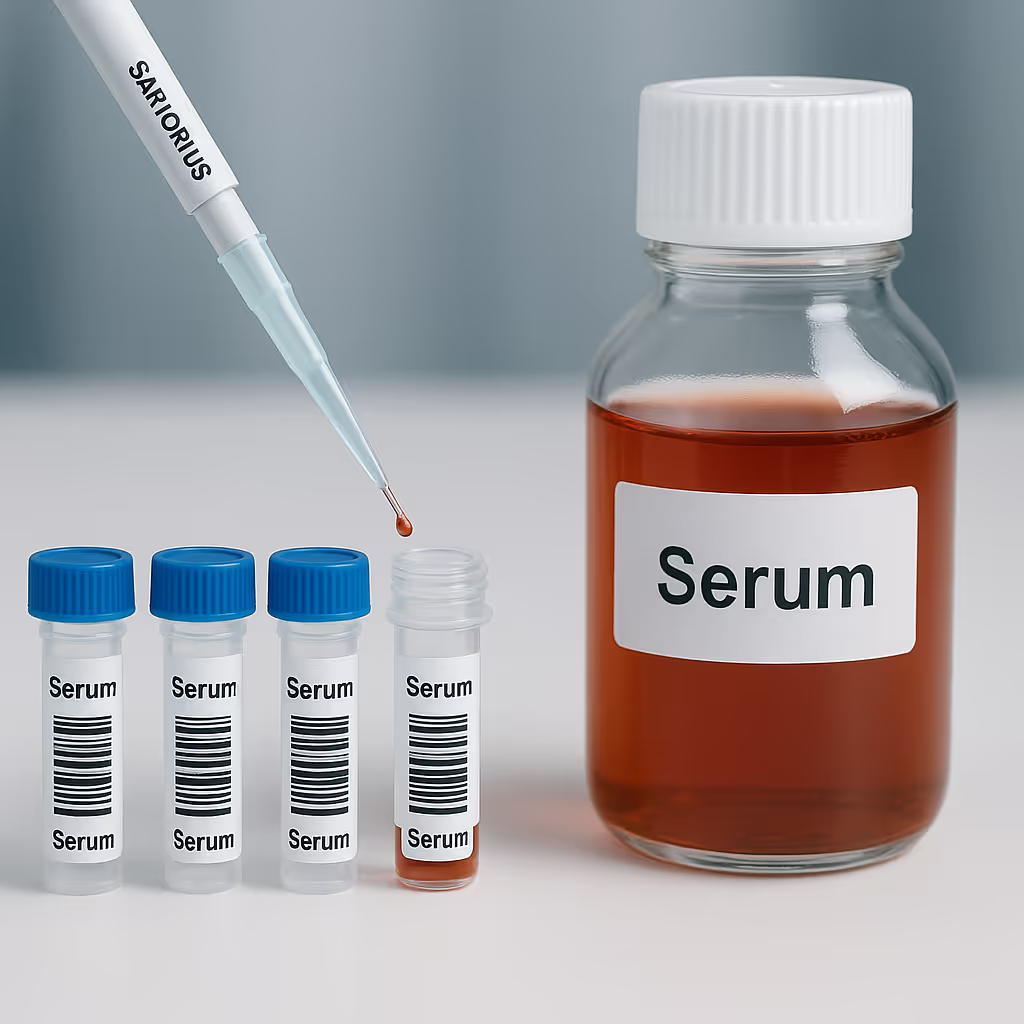
Samples are carefully aliquoted into vials or bottles (glass/HDPE) or other approved packaging material based on the requirement of clients before shipping them.
.svg)
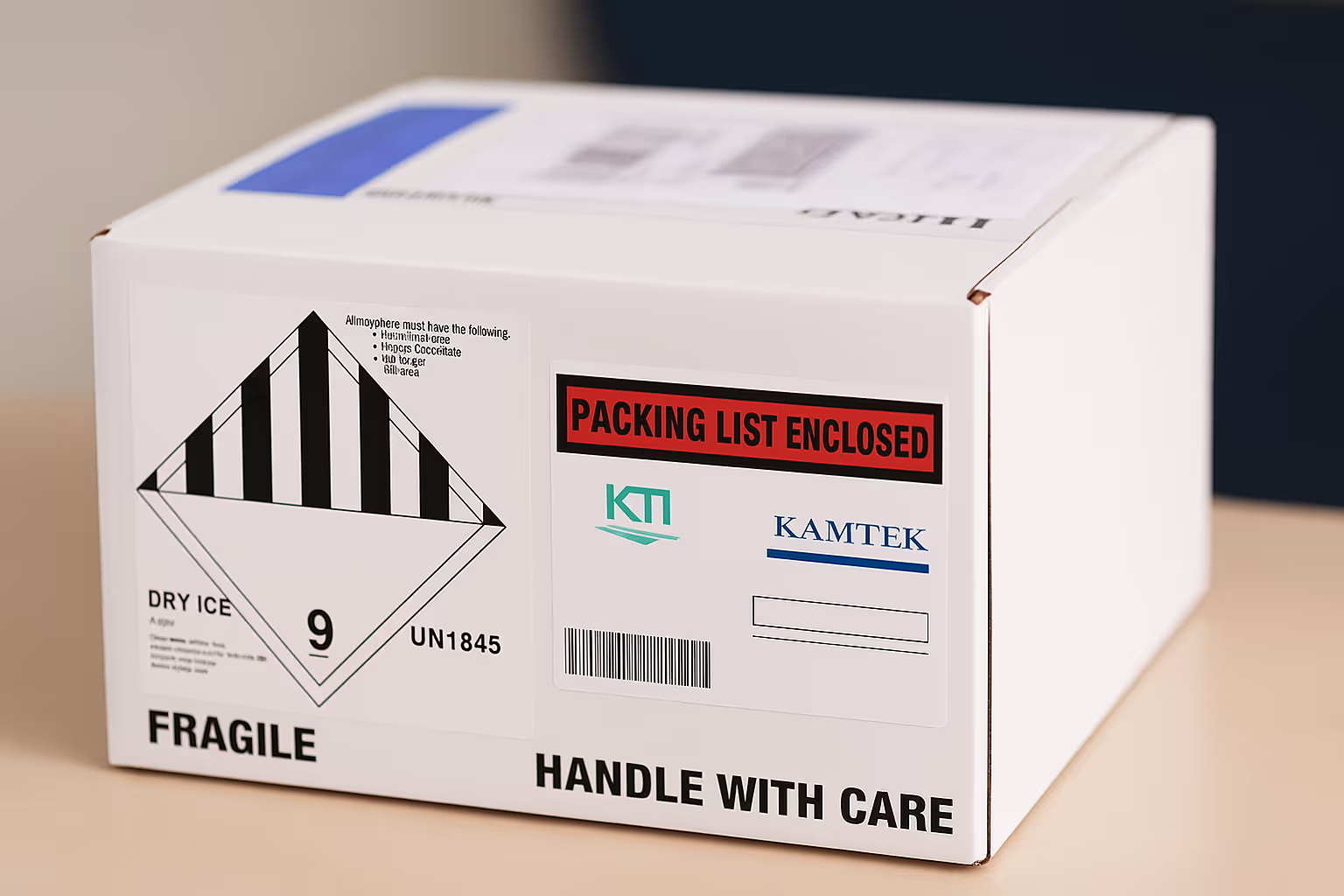
Each shipment is packed using KamTek-approved, UN-certified containers for respective temperatures (dry ice, cold packs, credo cube boxes, dry shippers). Proper labeling and documentation are affixed as per regulatory and international requirements.
.svg)
Temp. data loggers are added for critical shipments to provide real-time or downloadable temperature data.

.svg)
Shipments are coordinated via the appropriate carrier—FedEx, World Courier, same-day courier, or hand delivery—based on location and urgency.

.svg)
KamTek tracks every shipment to delivery. Clients receive full documentation, including shipment forms, tracking info, and signed receipt confirmation from the recipient.
Learn why SignPath Pharma chose KamTek as their trusted logistics partner for Phase 1 Glioblastoma clinical trial drug shipments requiring -20°C cold chain precision.
.svg)
.svg)
KamTek offers secure storage across multiple temperature ranges: room temperature (RT), 4 °C (refrigerated), –20 °C, –80 °C (ultra-low), and liquid nitrogen vapor phase (–170 °C to –196 °C).
.svg)
We operate a fully regulated, audit-ready environment:
1. FDA 21 CFR Part 211 / cGMP-compliant facility.
2. ISO 9001:2015 certified quality system.
3. Electronic records & signatures (21 CFR Part 11) for tracking sample custody.
4. Continuous monitoring of storage environments with redundant alerts and backup power.
.svg)
Upon receipt:
1. Samples go through a receipt workflow and are accessioned, logged into our secure inventory system.
2. Storage location is defined (based on chosen temperature tier) and chain-of-custody initiated.
3. Environmental conditions are monitored continuously; any excursions trigger automated alerts and client notifications.
.svg)
Yes. Clients retain full ownership and access to their stored materials. We sign a mutual NDA protecting your IP. You can schedule retrievals or shipments as needed. All withdrawals are documented through our chain-of-custody system and we coordinate logistics (on-site pickup or shipment) as requested.
.svg)
We provide:
1. Inventory lists with sample identifiers, storage status, and location mapping.
2. Environmental logs and audit reports upon request.
3. Optional certificate of storage verifying conditions, start date, and sample status for your regulatory records.
.svg)
Our facility is designed for resilience:
1. Backup power (generator/UPS) ensures continuous operation of freezers and LN₂ systems.
2. Disaster recovery protocols are tested and practiced regularly.
3. 24/7 facility monitoring, intrusion alarms, and environmental sensors alert staff immediately of any deviation.
.svg)
We support a wide range of clients—pharmaceutical companies, biotech firms, research institutions, government agencies—and handle materials including: cells, tissues, microbiological samples, diagnostics materials, frozen biobanks, reagents, and more.
.svg)
Pricing depends on multiple factors: sample volume/quantity, desired storage temperature tier, retrieval frequency, special handling requirements (biosafety level, containment), and any value-added services (aliquoting, barcoding, shipment logistics). We provide a formal quote after scope definition.
When timing, temperature, and traceability matter, KamTek delivers. Partner with us for seamless, compliant specimen shipments anywhere in the world.