If your study or product requires precision and compliance, we can build a kit for it. We build kits that support a wide variety of applications, including:
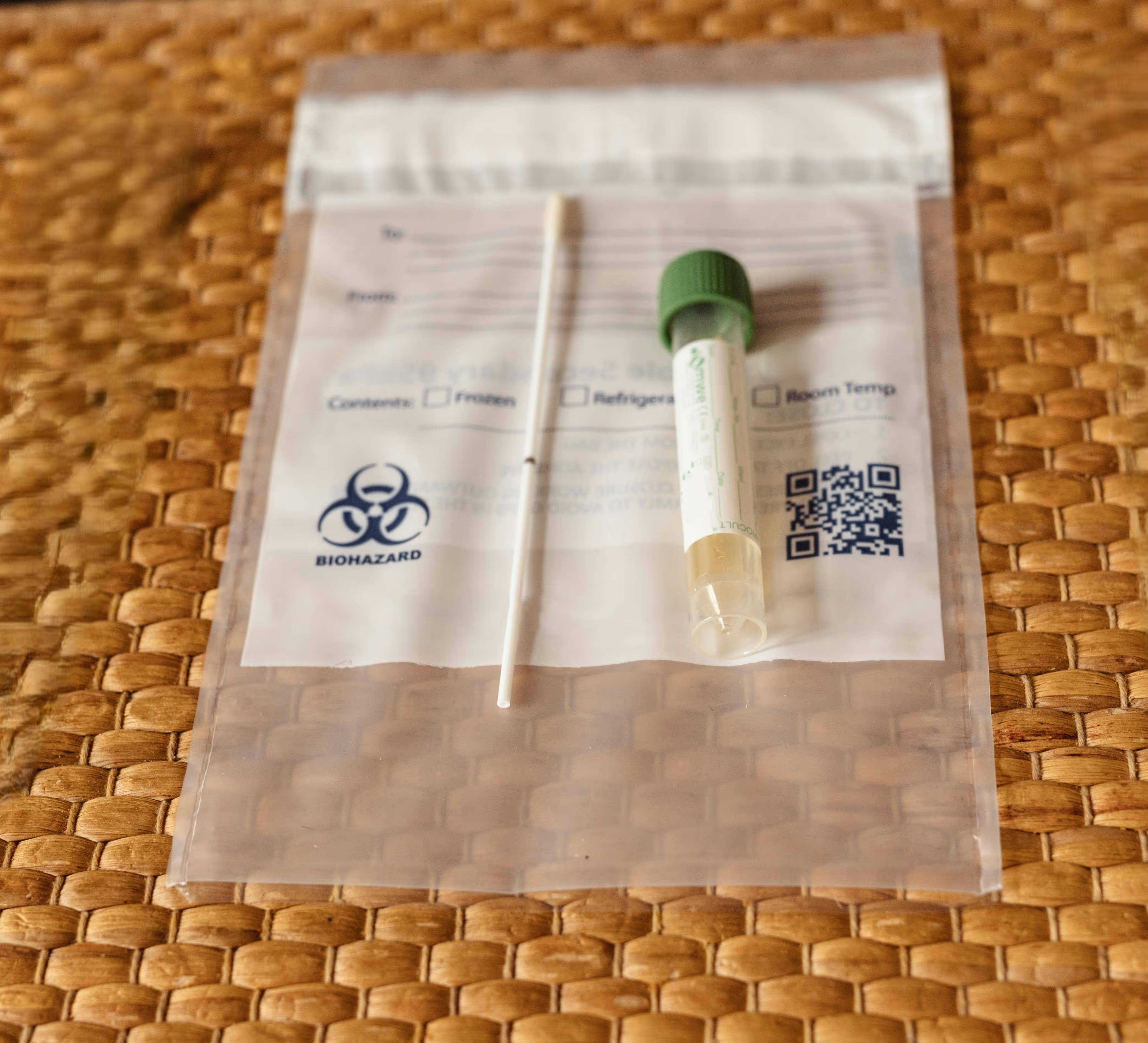
Kits with swabs, vials, blood tubes, forms, instructions, and return labels.
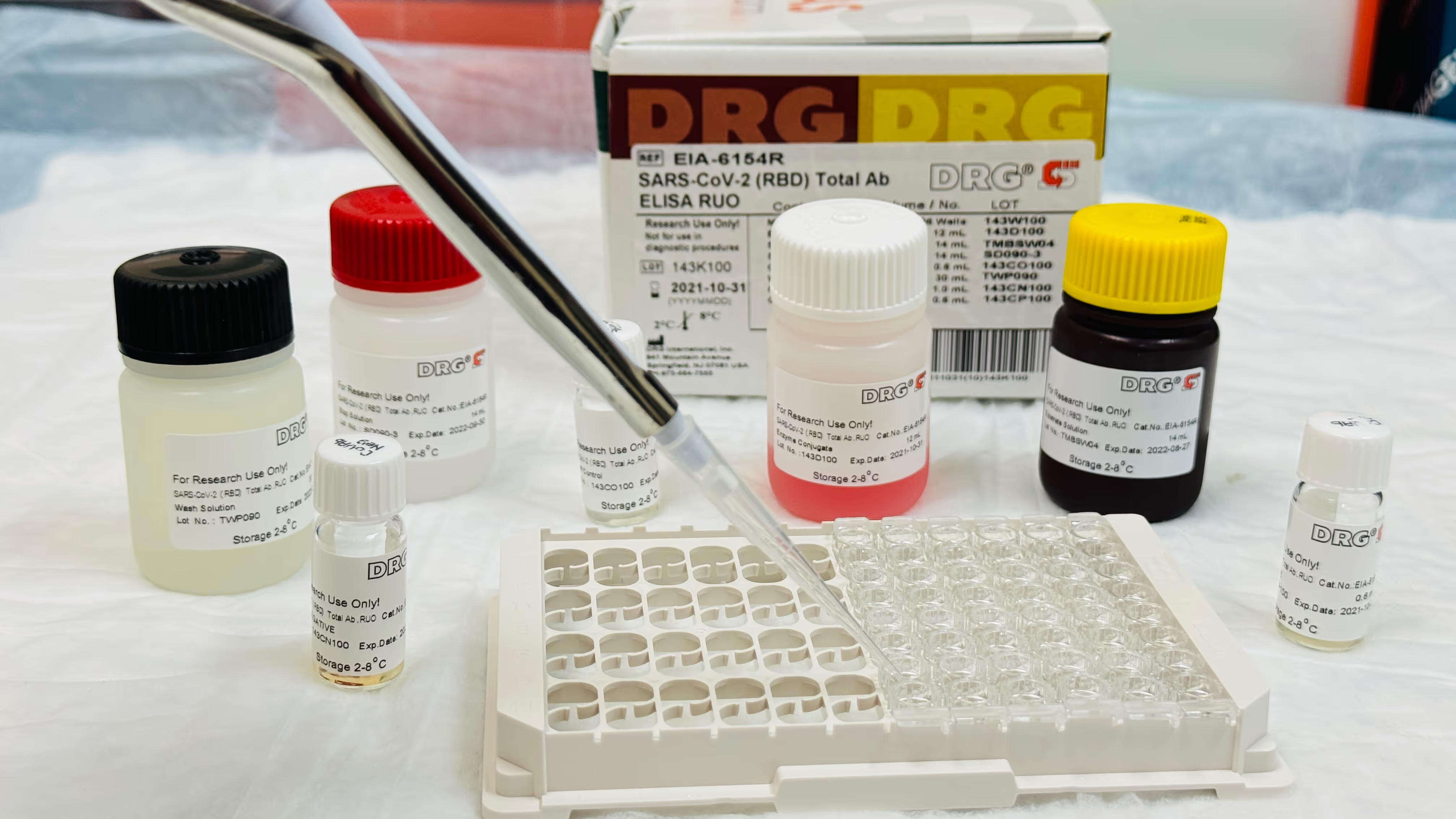
Kits with buffers, enzymes, tubes, and labels.
Kits with PCR reagents, antibodies, slides, pipettes, tips and return labels.

Kits with DNA/RNA extraction tools, transport media, instructions and return labels.

Kits with slide boxes, labeled cases, staining solutions, forms and instructions

Kits with investigational product vials, dosing tools, chain-of-custody forms, instrctions etc.

.svg)
We consult with your team to design kits fully tailored to your protocol or SOPs, with sterile or non-sterile options.
.svg)
Products are received, inspected, and logged into our secure system with full chain-of-custody documentation.
.svg)
Kits and individual components can be labeled with sponsor-defined barcodes or blind codes fully tracked in our CFR part 11 compliant custom inventory management system shared with you.
.svg)
Every kit includes instructions for use (IFUs), chain-of-custody forms, return labels and regulatory inserts alomg with other requested forms.
.svg)
Real-time inventory tracking, expiration management, and batch control for all kit parts and periodic updates to clients.
.svg)
Random QC checks are performed at every step to ensure kit accuracy and integrity and also before shipping to end users.
.svg)
Kits can be stored at dedicated controlled temperatures (RT to -196°C) and shipped globally with full logistics support.
.svg)
Streamlined re-supply options for long-term studies or recurring R&D operations
We support domestic and international kit distribution, including:
Your kits arrive exactly where and how you need them—on timeand protocol-ready.
.svg)
KamTek offers secure storage across multiple temperature ranges: room temperature (RT), 4 °C (refrigerated), –20 °C, –80 °C (ultra-low), and liquid nitrogen vapor phase (–170 °C to –196 °C).
.svg)
We operate a fully regulated, audit-ready environment:
1. FDA 21 CFR Part 211 / cGMP-compliant facility.
2. ISO 9001:2015 certified quality system.
3. Electronic records & signatures (21 CFR Part 11) for tracking sample custody.
4. Continuous monitoring of storage environments with redundant alerts and backup power.
.svg)
Upon receipt:
1. Samples go through a receipt workflow and are accessioned, logged into our secure inventory system.
2. Storage location is defined (based on chosen temperature tier) and chain-of-custody initiated.
3. Environmental conditions are monitored continuously; any excursions trigger automated alerts and client notifications.
.svg)
Yes. Clients retain full ownership and access to their stored materials. We sign a mutual NDA protecting your IP. You can schedule retrievals or shipments as needed. All withdrawals are documented through our chain-of-custody system and we coordinate logistics (on-site pickup or shipment) as requested.
.svg)
We provide:
1. Inventory lists with sample identifiers, storage status, and location mapping.
2. Environmental logs and audit reports upon request.
3. Optional certificate of storage verifying conditions, start date, and sample status for your regulatory records.
.svg)
Our facility is designed for resilience:
1. Backup power (generator/UPS) ensures continuous operation of freezers and LN₂ systems.
2. Disaster recovery protocols are tested and practiced regularly.
3. 24/7 facility monitoring, intrusion alarms, and environmental sensors alert staff immediately of any deviation.
.svg)
We support a wide range of clients—pharmaceutical companies, biotech firms, research institutions, government agencies—and handle materials including: cells, tissues, microbiological samples, diagnostics materials, frozen biobanks, reagents, and more.
.svg)
Pricing depends on multiple factors: sample volume/quantity, desired storage temperature tier, retrieval frequency, special handling requirements (biosafety level, containment), and any value-added services (aliquoting, barcoding, shipment logistics). We provide a formal quote after scope definition.
Partner with KamTek to design, assemble, and deliver custom kits with unmatched precision and compliance. From clinical trial support to complex research workflows, we make your operations smoother — one kit at a time.