Send us your freezer, pallet, or box — and we’ll send it back or store in house fully inventoried with digital records and a detailed manifest.
Want to create an inventory of bio samples at your site, we can send our staff to help you in this process.
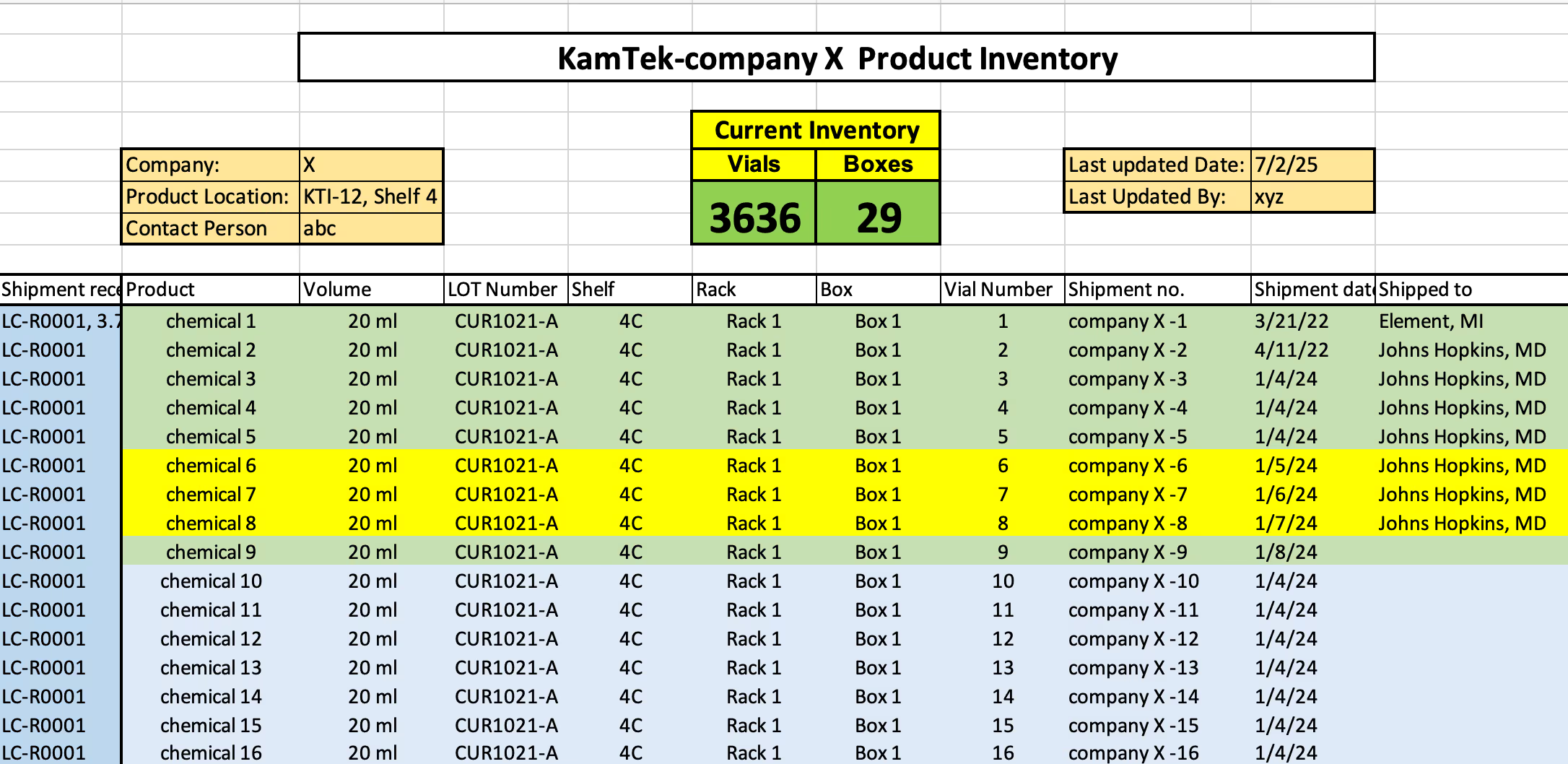
Capture of sample ID, format, type, volume, matrix, origin, and any custom metadata.
Organized, export-ready manifest in Excel, CSV, or custom formats for your LIMS.
Validation or replacement of existing labels with internal barcoding as needed.
Capture of slide ID, staining info, block case ID, tissue type, and related notes.
We send the inventoried data securely via encrypted file transfer or external drive—with or without the samples returned.
Spot-checking and audit logs to ensure 100% accuracy.

.svg)
The process begins with a consultation to understand your inventory needs — what types of samples you have, the level of detail required, and any deadlines or regulatory requirements.
Our team drafts a custom inventory protocol that defines how your materials will be handled, verified, and recorded

.svg)
We receive your materials, whether in a freezer, pallet, or shipment box. Each item is checked for condition, chain-of-custody logged, and staged for storage and inventory.

.svg)
Samples are carefully withdrawn from their storage locations using validated 'specimen withdrawal containers' maintained at the optimal temperature required for the samples (RT/4oC/-20oC/-80oC/LN2).

.svg)
Our team create a custom inventory template tailored to your project—typically including fields like Sample ID, Box ID, Location, Sample Type, Volume and Comments—for easy integration with your systems. Our technicians verify each sample manually or via barcode scanning at controlled temperatures, ensuring accuracy, resolving label issues, and capturing missing metadata.

.svg)
We conduct a final review of all inventory data — verifying accuracy across Excel sheets or databases, checking for duplication, mismatches, or incomplete entries.
.svg)
You receive a complete encrypted inventory report including:
-Verified and formatted data files
-Sample counts and locations (freezer, box, rack, row)
-Notes on any discrepancies or damaged/missing samples
-Chain-of-custody documentation if required
Your inventory is now clean, traceable, and ready for audits, research, or downstream workflows.
.svg)
After inventory, samples are either stored at KamTek for long term use or shipped back to the Client under strict chain of custody and tracebility.

From frozen vials and FFPE blocks to complex clinical shipments and decades-old slides, we’ve seen—and inventoried—it all.
No one-size-fits-all. We develop project-specific inventory workflows to match your materials, goals, and compliance needs.
We don’t rely on off-the-shelf tools. Our proprietary systems manage millions of data points securely and efficiently—customized to your structure.
We maintain temperature and custody controls throughout the entire inventory process, using validated withdrawal containers and secure handling procedures.
Leading institutions rely on KamTek for high-stakes inventory management—from NIH and FDA programs to clinical trial sponsors and top-tier biotech firms.
Partner with KamTek for accurate, secure, and fully traceable sample inventory—whether it's one box or an entire biobank.