Abstracts & Posters

In vitro and In vivo activity of Clofazimine against Clostridium difficile
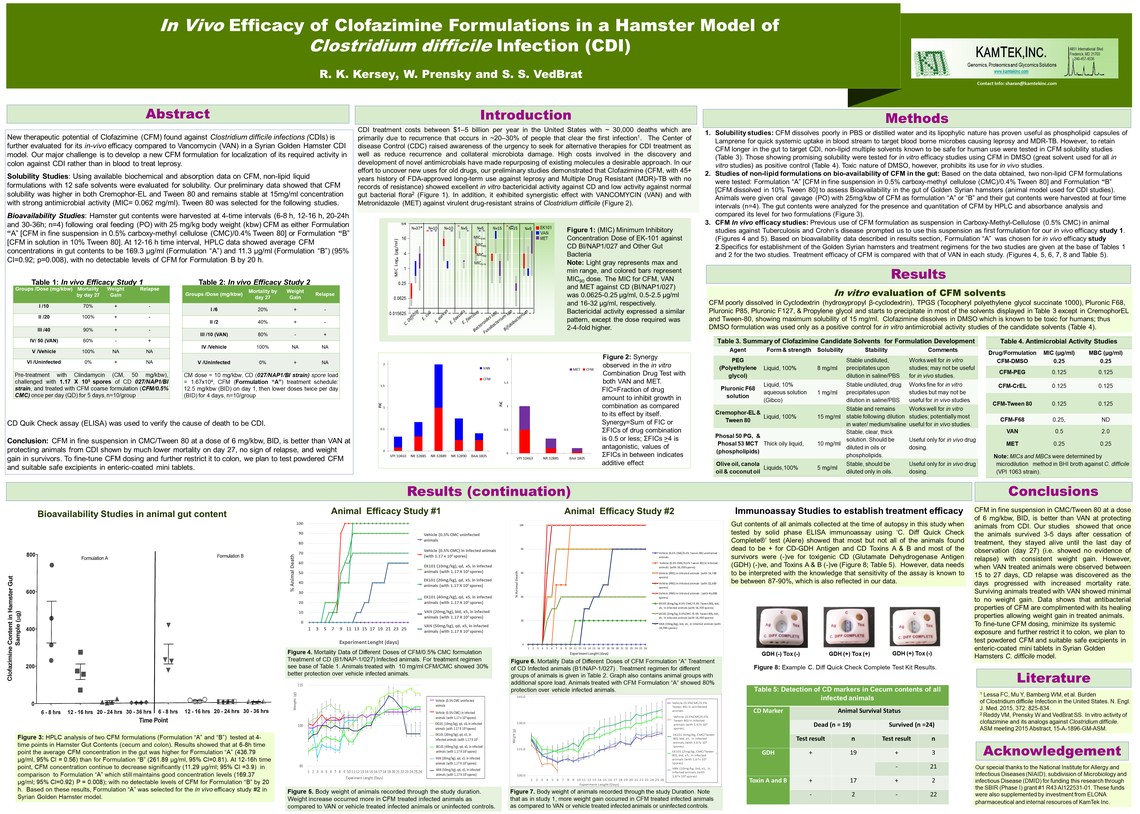
R. K. Kersey, W. Prensky, S. VedBrat
KamTek is repurposing Clofazimine (CFM), a therapeutic agent for leprosy and MTB-DR, to treat Clostridium difficile (CD) infections, the root cause of ~30,000 annual deaths in the USA. Our Minimum Inhibitory Concentration (MIC) studies with CFM continues to exhibit both greater potency against CD strains (MIC 50-MIC90 as 0.0625-0.25 µg/ml) than other currently used antibiotics (Vancomycin, VAN: MIC 50-MIC90 as 0.5-2 µg/ml and Metronidazole, MET: MIC50-MIC90 2-4 µg/ml), and more specificity against CD than other gut bacteria (MIC 50-MIC90 as 4-8 µg/ml). Here, we report synergistic activity of CFM-MET, and CFM-VAN pairs against CD strains, with 8 and 16-fold decrease in MICs for CFM and MET, and 4 and 2-fold for CFM and VAN, respectively. Solubilized CFM aqueous formulations (CFM/PEG, CFM/CrEL, and CFM/Tween 80) show activity against CD (MIC50-MIC90 as 0.125-0.25 µg/ml) similar to that of standard CFM/DMSO. Bioavailability studies of CFM/CMC/Tween 80 and CFM/Tween 80 aqueous formulations in hamsters’ gut content harvested at 4-time intervals indicated that CFM in the first formulation had higher average concentration (169.3 mg/ml) than the second one (11.3 mg/ml) at 12- 16h interval, with almost no detectable levels by 20 h. In vivo efficacy studies of CFM in a Hamster model of CDI were performed with 2 formulation types. Study 1: Clindamycin (CM) treated animals (50 mg/kg) were challenged with 117,000 CD spores, and PO with CFM/CMC [10, 20 and 40 mg/kg doses, qd (5 days)]. Animals treated with VAN (50 mg/kg, qd, 20 mg/kg, bid), and vehicle (infected and non-infected) served as controls. The 10 mg/ml CFM/CMC treated animals showed 30% better protection over vehicle infected animals. Study 2: CM-treated animals (10mg/kg) were challenged with 16,700 CD spores, PO with 3 doses of CFM/CMC/Tween 80: 6, 4 and 2mg/kg, bid, for 4 days after first day treatment with 12.5mg/kg, bid), VAN-treated animals received 10mg/kg, PO, bid. The 6mg/kg treated animals showed 80% protection over vehicle infected animals, meanwhile the 4 and 2mg/kg treated animals showed 20% and 40% protection, respectively. Contrary to VAN treated animals, protected animals in both studies showed no sign of relapse during 27-day observation time. In addition, 20% increase in weight was observed in animals treated with CFM as opposed to VAN. We conclude that in vivo efficacy studies have to be done using CFM in solid oral dose formulation as enteric coated capsules (EK101 formulation) to be delivered directly to colon to be evaluated for most optimal therapeutic value with promise of avoiding relapse.

In vitro Activity of Clofazimine and its Analogs Against Clostridium difficile
V. M. Reddy, W. Prensky, S. S. VEDBRAT
Hospital-associated infections (HAI) due to Clostridium difficile (CD) are on the rise. With the emergence of hyper-virulent CD strains there has been a significant increase in the morbidity and mortality, particularly among the elderly population. At present there are limited antimicrobial agents available for treating CD infections. While searching for new use for old drugs in our inventory –repurposing- we discovered excellent in vitro activity of clofazimine (CFM) and several of its analogs against CD. Initially we screened 13 clofazimine analogs in vitro against CD and identified four compounds (CFM, B746, B4157 and B4129) with highest activity. Subsequently, the in vitro activity (MIC) of these four compounds was determined against large number of CD strains (n=36), by broth microdilution method using brucella broth, in comparison with vancomycin (VAN) and metronidazole (MET). The in vitro activity of all the four CFM analogs was significantly superior to VAN and MET (Table). The MIC90 of CFM and B746 (0.25 μg/ml) were 8- and 16-fold lower than that of VAN and MET, respectively. Analogs B4157 and B4129 were most active with MIC90 2-fold lower than that of CFM and B746. CFM was found to be bactericidal with MBC/MIC ratio of 1-2 and was synergistic in combination with VAN against some CD strains. When tested for in vitro activity against normal intestinal flora: all the four analogs were inactive against E.coli (MIC >32 μg/ml). The in vitro activity of CFM and its analogs against S.aureus, Enterococci and Bacteroides spp. was modest and varied significantly (MIC 0.25 – >32 μg/ml). Thus the in vitro data obtained so far in this study indicate high therapeutic potential of CFM against CD infections. Clofazimine, a riminophenazine, was originally developed against tuberculosis (TB), presently being used against lepromatous leprosy, multi-drug resistant-TB and M.avium complex disease. Because of its anti-inflammatory and immunomodulatory activities, it is also being used in treating Crohn’s disease and several other immune mediated diseases of the skin and mucous membranes. With its long history of use and well known pharmacological and toxicological features the time required and cost involved for introduction of CFM into clinic will be greatly reduced.