During the peak of the COVID-19 pandemic, one of our large biorepository client engaged KamTek to address a critical need: converting over 1,000 individual vials of human convalescent plasma from recovered COVID-19 patients into serum for research and therapeutic development. The primary challenge was to develop and validate a defibrination process that preserved COVID-19 antibody titers, while enabling large-scale processing, aliquoting, and compliant shipment to client’s facility.
The project required
KamTek first performed a series of controlled experiments using healthy human plasma to determine the optimal Thrombin/CaCl₂ concentrations, incubation conditions, and centrifugation parameters. This was performed to ensure that the process works before working on the precious patient samples.
Key Findings:

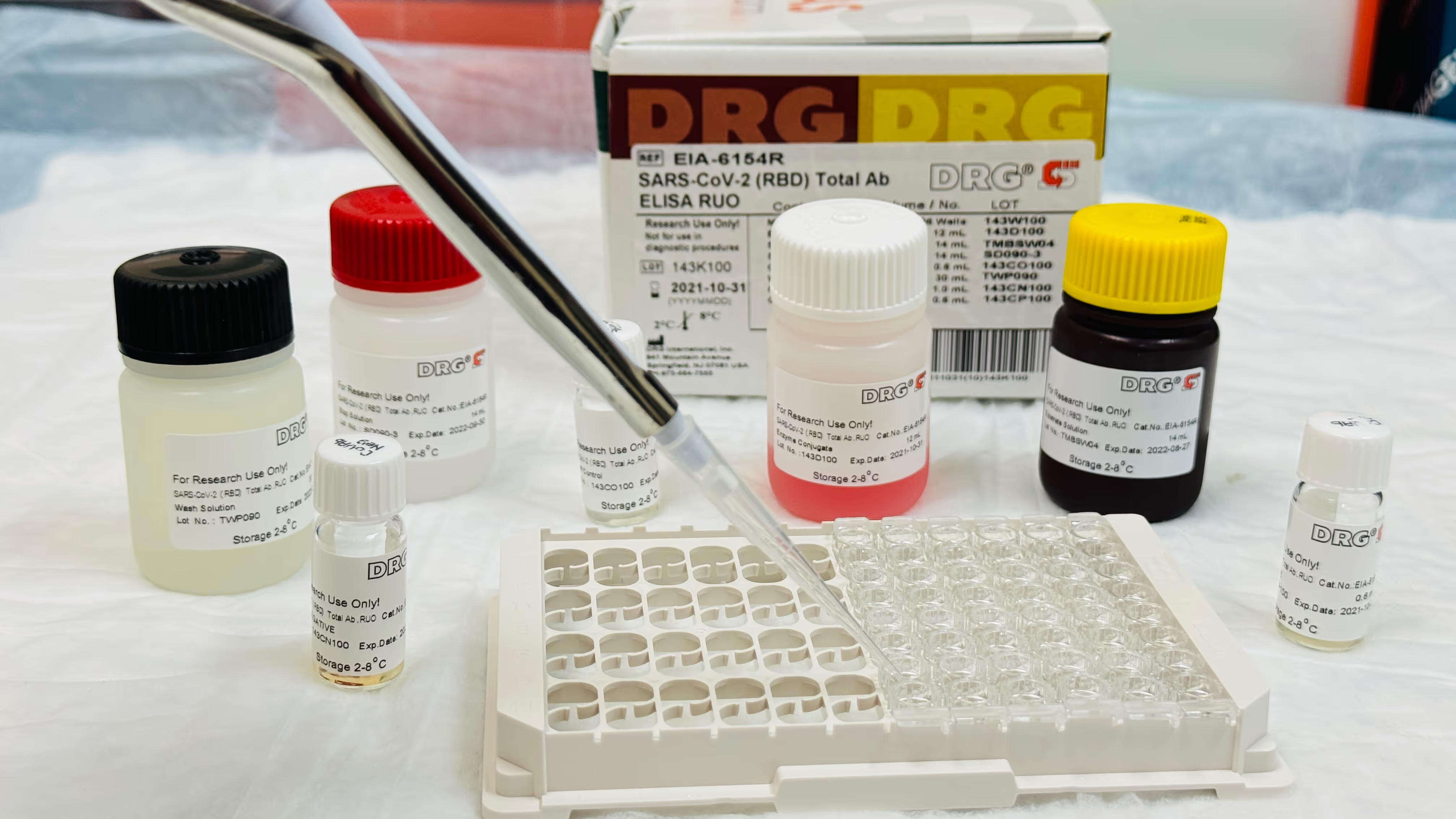
Before bulk processing, KamTek tested the optimized method on a single vial of convalescent plasma received from the client. Pre- and post-defibrination antibody titers were determined using ELISA and compared, confirming no adverse effect on COVID-19 antibody levels.
KamTek processed 1,001 plasma samples in four batches
Each batch was:
Total Serum Recovered: ~ 2.6 liters (combined yield from all four batches) from 2.75liters of plasma.

COVID-19 antibody titers were retested for each processed batch by ELISA test. Results confirmed no significant difference between pre-and post-defibrination samples, validating the process integrity.
Pooled serum was aliquoted into 1,000 labelled cryovials (250 µL each) using a repeater pipettor and the remaining serum was divided into 51 bulk tubes (45 mL each). All aliquots were stored at -80°C and shipped to client on dry ice, with full chain-of-custody and temperature compliance.
KamTek successfully delivered a validated, scalable, and compliant solution for Client critical COVID-19 serum project. The processed samples, with preserved antibody activity, provided a valuable resource for research and therapeutic development during the pandemic.
.svg)
KamTek offers custom biospecimen processing, aliquoting, and compliant sample logistics to support research and clinical programs. Contact us today to discuss how we can assist with your complex sample processing needs.