


Established in 1992, KamTek is dedicated to advancing biomedical research through innovation, quality, and excellence. Our specialized expertise includes Cryopreservation and Cryo-Storage, Molecular Biology, Microbiology, Cellular Services and Logistics. For over 30 years, our primary focus has been preserving the integrity and identity of biospecimens, earning us the trust of researchers, clinical institutions, and regulatory agencies worldwide.
Our vision remains clear: to empower scientific breakthroughs that enhance human health and alleviate suffering.
.svg)
Handled blood, urine, human cells, mouse cells, microorganisms, BSL 1 and BSL 2 samples, pathology slides and blocks, antibodies, DNA, RNA, and many more
.svg)
A seasoned team of scientists, including numerous PhDs, with deep expertise across research, clinical, and regulatory environments.
.svg)
Trusted long-term partner of leading institutions, including over two decades of collaboration with the FDA and NIH.
.svg)
Regularly audited by external orgs and clients to ensure compliance, quality, and efficient operaitons.

At KamTek, we specialize in Biorepository Operations and Laboratory Services designed to support our clients' research and development efforts.

Receiving, storing, managing, and shipping samples across various temperature ranges (Room Temperature, 4°C, -20°C, -80°C, -170°C,and -196°C).
.svg)

Advanced on-site laboratories for sample processing, testing, and research—including molecular biology, microbiology, and cellular services.
.svg)
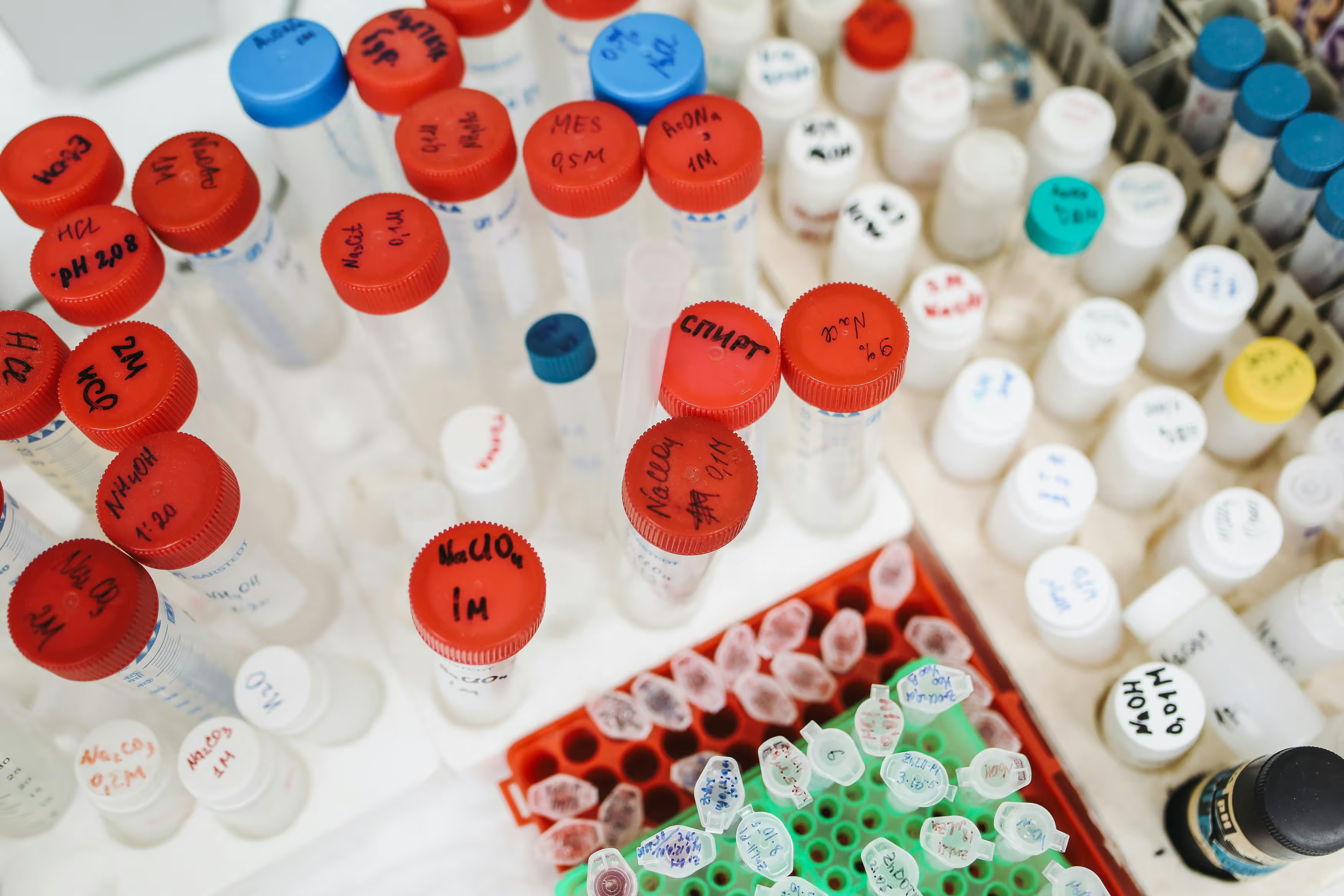
End-to-end tracking, labeling, barcoding, accessioning, and inventory control with full chain-of-custody and regulatory compliance.
.svg)
Strategically located in Maryland's I-270 Technology Corridor, KamTek is conveniently situated within one hour of prominent research and regulatory institutions, such as the FDA, NIH, Johns Hopkins University, Walter Reed National Military Medical Center, NCI, and numerous private research organizations.
Sample receipt and shipping stations, Cold storage units, power and data backups
Advanced microbiology, cell culture, molecular and sample processing laboratories
Facility designed in accordance with FDA cGMP guidelines (21CFR Part 211)
Facility equipped with burglary alarm, surveillance cameras, environmental monitoring, instant notifications, 24/7 staff on call


Average Client Rating: 4.83 out of 5.00
Net Promoter Score (Customer Loyalty): 9.6 out of 10.0
CPARS Ratings: Multiple satisfactory assessments
Client Audits: Consistently completed with zero non conformities
Our track record speaks volumes, demonstrating reliability, trust, and unwavering commitment to our clients' success.
Henry Jackson Foundation (HJF)
Walter Reed
Exxon Mobil
HHV-6 Foundation,
Hudson
Silbiotech
Texcell
Insulin Club
SignPath
VASTEC
George Washington University
Clarent Biopharma Inc
NIH-NIDCD
NIH-NCATS
Roche Holding AG
Diazyme Laboratories Inc
LB Pharmaceuticals Inc
Creative Biopeptides Inc
Johns Hopkins University
ATCC
DoD-VHC
US Army-JPC
NIH-NIAID
NIH-NEI
NIH-NICHD
NIH-OD
Leidos
Corning
FDA
NIH








































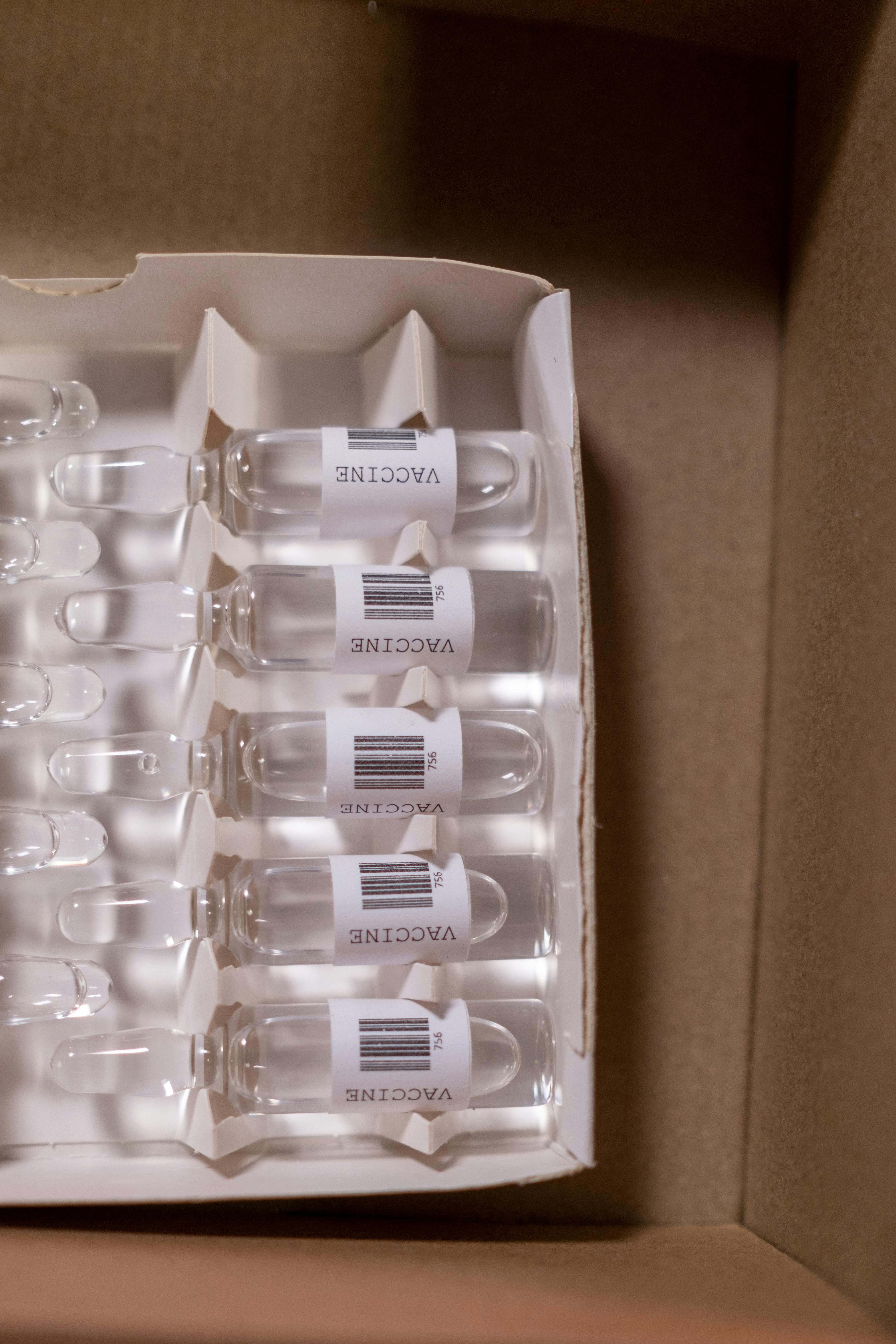
Supporting drug discovery, development, and clinical trial logistics through advanced kitting, sample processing, storage and regulatory-compliant services.

Partnering with biotech companies in translational research, biomarker discovery, microbiome studies, secure storage and molecular diagnostics.
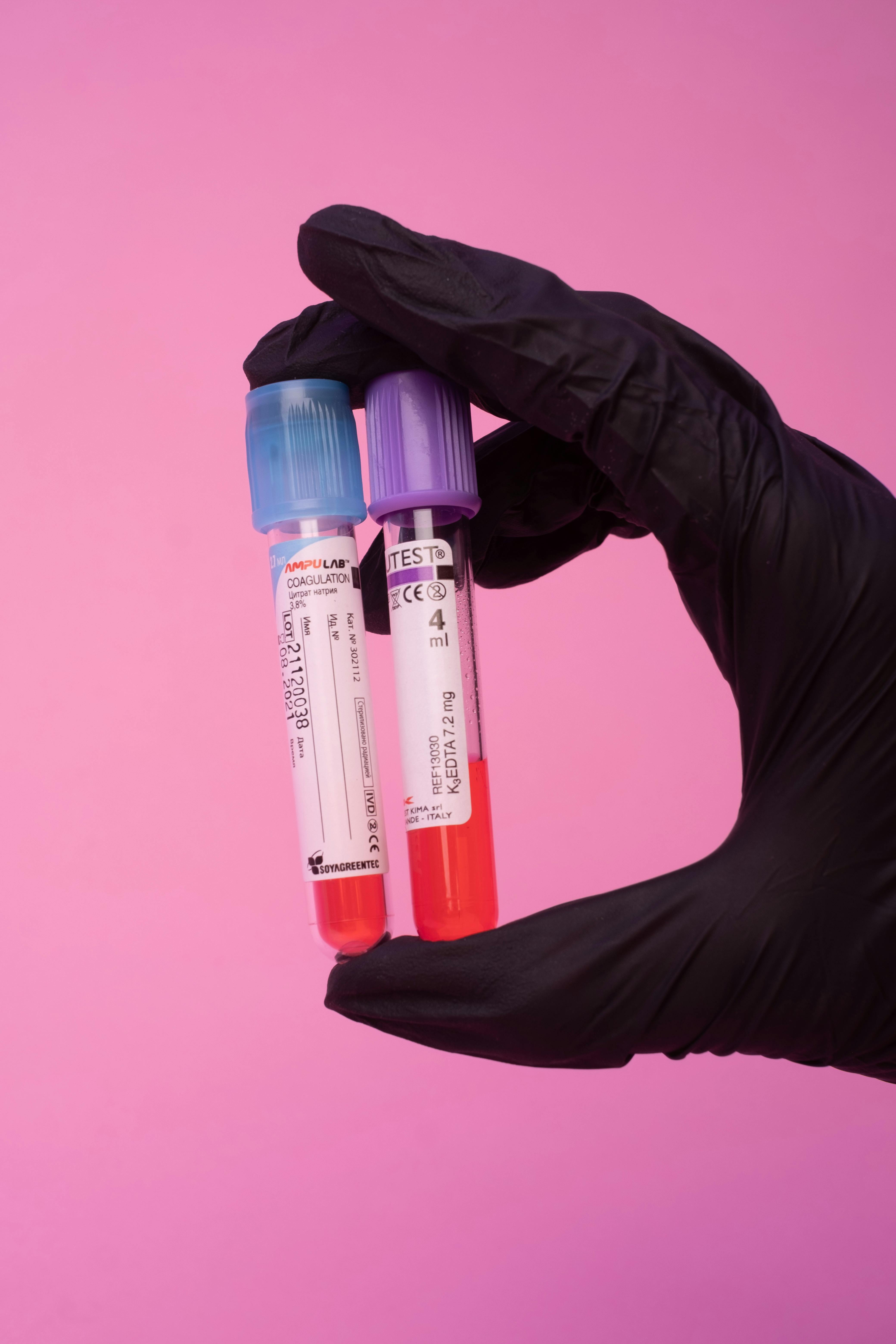
Providing reliable offsite storage, sample management, and processing solutions for high-throughput clinical environments with regulatory compliance.

Experienced in handling sensitive medical archives and pathology collections for federal clients, secure storage and maintenance of research material.

Empowering universities and research hospitals with lab services, aliquoting, microbiology support, and compliant biospecimen handling.
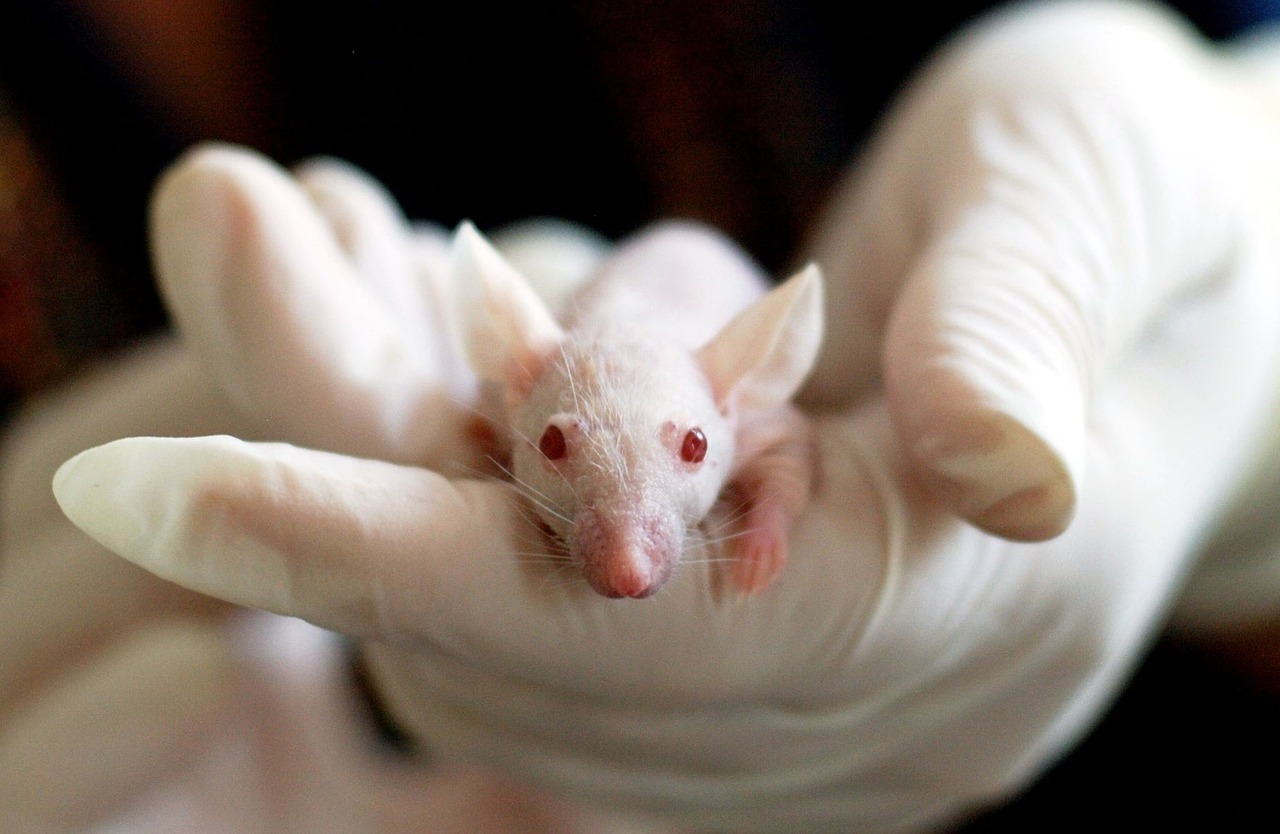
Serving contract research and manufacturing organizations with scalable lab support, logistics, and data integrity services across development phases.

Offering specimen handling, secure storage, and analytical support to programs addressing infectious diseases and population health.
Whether it’s biospecimen management, secure storage, lab services, or compliant logistics—KamTek is your reliable partner in life sciences and beyond.